Particle motion The particles in a gas are moving very quickly in random directions. -Has a definite volume but no definite shape.
2019 Aqa Physics Molecules And Matter Pressure In Gases Teaching Resources
This process in which particles move from an area of high concentration region A to an area of low concentration region B is called diffusion.

. Gas particles move in random directions this sort of movement is call Brownian motion gas particles are very open and have a lot of space to move around unlike solids. Does temperature not affect the movement of particles. Here R equals about 8314 KPaL molK diavinad8 and 6 more users found this answer helpful.
2 on a question 1. From this it can be seen that an increase in temperature causes an increase in the kinetic energy of the particles in an ideal. Gas In a gas particles are in continual straight-line motion.
Describe the motion of particles in a liquid when its heated. Particle motion The particles in a gas are moving very quickly in random directions. Know how to determine the formula of a metal oxide by combustion eg.
They are considered dimensionless points. PV nRT is the relation that relates the pressure of a gas P in KPa its volume V in L its quantity n in mol and its temperature in K. All of the particles on each states of matter vibrates but moves a little except gas Describe how the motion of particles are related to the pressure exerted by the gas.
The kinetic energy of the molecule is greater than the attractive force between them thus they are much farther apart and move freely of each other. The particles decrease in speed. Describe the motion of particles in a solid when its heated.
The kinetic energy of the molecule is greater than the attractive force between them thus they are much farther apart and move freely of each other. Molecules move faster when temperature increases and move slower when. Magnesium oxide or by reduction eg.
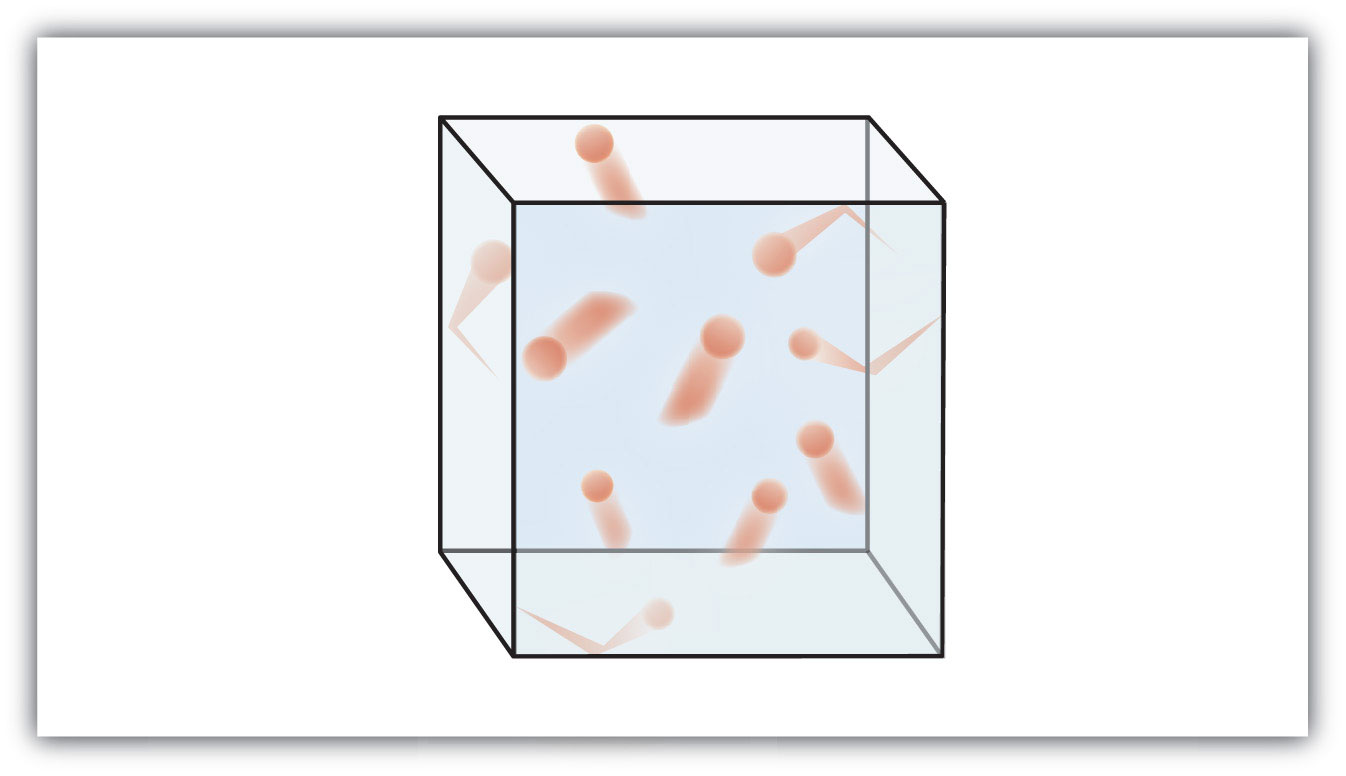
Gas molecules are in rapid random motion. Particles in a gas are far apart and can move about freely Describe the motion of particles in a solid when its heated When the particles of a solid are heated they move faster move farther apart and take up more room. This means that a gas has nothing to hold a specific.
An increase in total kinetic energy An increase in the speed of individual particles The total kinetic energy of an ideal atomic gas is given by 32Nk_BT where N is the number of atoms k_B is the Boltzmann constant and T is the temperature. -Has a definite shape and volume. The motion of the particles of liquid is slightly slippery and is fluid.
The more energy a particle. In most cases there are essentially no attractive forces between particles. Gas In a gas particles are in continual straight-line motion.
As the temperature of a solid liquid or gas increases the particles move more rapidly. The speeds of the particles vary but on average they move quicker than they do in liquids and solids. Particles can move all over the place spread far apart.
Arrenhasyd and 5 more users found this answer. 135 Triple only understand how to carry out calculations involving gas volumes and the molar volume of a gas 24dm³ and 24000cm³ at room temperature and pressure rtp 136 practical. First week only 499.
As the temperature falls the particles slow down. Describe the motion of the gas particles. An ideal gas is made up of.
Solution for Describe the characteristic movement of the particles of solid liquid and gas and arranged them in increasing motion of the particles. In a gas at room temperature the particles of the gas move at a very high speed and in a random manner. It is because of the kinetic energy of the liquid particles which is more than solids and less than gas particles.
Over time what is happening. Temperature directly affects the movement of particles such as molecules. Describe the motion of gas particles.
Particles in a gas are far apart and can move about freely Describe the motion of particles in a gas When the particles of a solid are heated they move faster move farther apart and take up more room. Particles roll over one another can move around some space between particles. Because the particles are in motion they will have kinetic energy.
The motion of the particles is increased by raising the temperature. The speeds of the particles vary but on average they move quicker than they do in liquids and solids. Conversely the motion of the particles is reduced by lowering the temperature until at the absolute zero 0 K the motion of the particles ceases altogether.
The molecules of a gas have insignificant volume compared to the distances between them. Particles are closely connected in a crystal lattice vibrate slightly on the spot. Start your trial now.
A gas has no definite volume and it is also assumed that there is minimal interaction between gas particles.
Ixl How Does Particle Motion Affect Gas Pressure 8th Grade Science

0 Comments